
Well, 18 months has elapsed since the start of the 18 month CBD Research Project we proposed and helped fund into childhood brain tumours and it certainly looks to have been worthwhile with more research continuing as a result – full report below:


In vitro evaluaton of the effect of cannabidiol as an adjuvant therapy for paediatric brain tumours: Update June 2019
The purpose of this research project is to conduct a range of experiments to explore how paediatric brain tumour cells react with CBD. Brain Tumour Action, the Astro Brain Tumour Fund, Amelia’s Appeal, Make William Well and the Jessica Hope Foundation are jointly funding a research technician and experiment costs.
Since our last update, we have again made progress with the research project and have been fortunate to have three MSc students who wished to be part of the project in an extended role. These students are all very committed and have tackled additional questions not part of the original application, but which have provided further insight.
We have continued to experience problems with the DIPG cell lines which have become infected when they are grown in antibiotic free media. Despite discussing with colleagues working with DIPG cell lines no solution has been found and we have been unable to move forwards with these cells at this time. However, we still have five cell lines which we are able to perform experiments on: SF188 and KNS42 (paediatric high grade gliomas), BxD-1425EPN and DKFZ (ependymomas) and normal astrocytes which are used as control cells.
Recently high impact journals have increased the number of experimental repeats required for publication from 3 to 6. We have therefore increased our experiments accordingly. The data we have obtained to date has been presented in poster format at a recent Nottingham Paediatric Roadshow Conference which allows research being performed locally to be celebrated. This poster will also be presented at the 2019 International Cannabinoid Research Symposium in the USA at the end of June. The research technician employed on the project presented his data to the Children’s Brain Tumour Research Centre Spring meeting in May 2019 which sparked lots of questions into the potential role of this therapy. The data has also been discussed with the NCRI Paediatric England trials group.
In our last report, we reported that after our experiments with CBD the cells die and we are now trying to elucidate the mode of cell death. There 2 main modes of cell death are so-called Apoptosis (programmed cell death) and Autophagy (self-eating/ self-destruction). In addition we wish to study the effect of CBD on the cells both in normal oxygen conditions and also when oxygen levels are limited. This is thought to better represent the 3-dimensional structures of tumours where the centre of the tumour is deprived of adequate oxygen supply and in the brain. To do this, we have used the hypoxic chamber for experiments. One of the successes over the last few months is that we have been able to maximise our use of the hypoxic chamber and perform lots of experiments in it. Below is a summary as to the status of all of the different experiments being performed in this study. For the purposes of this report, data from SF188 cells only are shown in the figures.
1. Metabolism Assay
Cells used: SF188 and KNS42 (paediatric high grade gliomas), BxD-1425EPN and DKFZ (ependymomas) and normal astrocytes which are used as control cells.
Metabolism assays are used to quantitatively measure the effect of a drug on the proliferation of cells i.e. how quickly the cells are growing and dividing. We have conducted a range of experiments so that we can extrapolate the concentration of the drug needed to achieve 50% cell death. The concentration of CBD which causes 50% cell death (EC50) had previously been confirmed in the SF188, BxD-1425EPN and astrocyte cells in both normal and hypoxic conditions as well as the 24 hour and 5 days’ time points. It has now also been confirmed in the KNS42 and HSJD07 cells with experiments continuing on the DKFZ and T8/18 cell lines. The figure below shows an example of the effect of increased CBD concentration on SF188 cell viability.




Figure 1
Figure 1 – Shows the results of metabolism assays on SF188 cells in both short term culture (24 hours) and long term culture (5 days) in both normoxic and hypoxic conditions. The EC50 decreased from 17.6µM at 24 hours to 14.8 µM at 5 days. The graphs in the lower panels show that the level of cell death is decreased under hypoxic conditions when compared to the cells under normoxic conditions (upper graphs). In fact an IC50 (50% cell death) is not achieved with 24 hours incubation in hypoxia. This implies that higher concentrations of drug may be necessary.
2. Western Blotting
Western blots are used to detect specific proteins within cells. The cells are cultured either alone, in the vehicle the drug is diluted in, in CBD or in the presence of two different control drugs, staurosporine (initiates apoptosis) or chloroquine (initiates autophagy). The cells are snap frozen at the end of the experimental time and then protein is extracted from the cell pellets. Western blot analysis is then used to identify which proteins have been released to allow the mode of cell death to be established.
At present we have optimised two of the four antibodies we intend to use to identify the mode of cell death (LC3B for autophagy and PARP for apoptosis). We have an MSc student who is running the Western blots on SF188, BxD-1425EPN and astrocytes using these antibodies. This work is due to be completed over the next month. We are currently optimising the other antibodies (P62 for autophagy and Caspase-3 for apoptosis). From the work completed to date there is strong evidence that there is increased expression of the autophagy marker, LC3B, 24 hours after exposure to CBD however, there was no increase in this marker after 5 days, indicating autophagy is the immediate mode of cell death. In contrast, the apoptosis marker, PARP, was expressed after in those culture left in the CBD media for 5 days but not after 24 hour exposure, indicating that both modes of cell death occur but at different time points. We have also observed stronger band staining as the concentration of CBD increases indicating there is an increase in the expression of both apoptosis and autophagy markers as the concentration of CBD is increased. Importantly, there was no evidence of either mode of cell death in the astrocyte cells exposed to CBD for 25 hours.

Figure 2
Figure 2 – Western blot showing SF188 cells grown in various drugs (C= cells alone, V = vehicle, 10 = 10µM CBD, EC = 17.6µM CBD at 24 hours and 14.8µM CBD at 5 days or 20µM, for 24 hours and 5 days. Antibody control cells were grown in sta = staurosporine for 4 hours or Chl = chloroquine overnight. Antibodies used were LC3B for autophagy (A), PARP for apoptosis (B) and GAPH as control (C). (D) Astrocytes were also evaluated with LC3B to ensure CBD was not causing cell death to a control cell line death in astrocyte cells exposed to CBD for 24 hours (D).
3. 3D Spheroids
3D spheroid culture is thought to better represent the growth of brain tumours in vivo. As previously described, in the CBTRC we have developed a model for culturing cells in 3D which allows end point evaluation by immunohistochemistry (IHC) a technique usually reserved for pieces of tissue. To date 3D spheroids of SF188, BXD-1425EPN and astrocytes have been cultured, processed and embedded in wax ready for a number of antibodies to be screened by IHC. We are using the same antibodies across our 3D spheroid culture and Western blot experiments, which means we are using different methods to validate the data. We will also investigate whether there is a change in the proliferation rate of the spheroids cultured in CBD using the antibody Ki67.
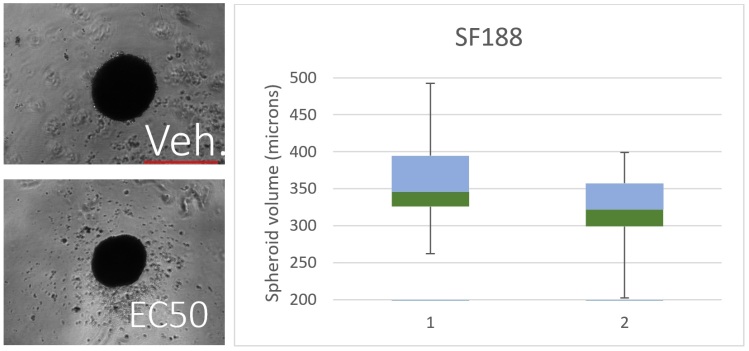
Figure 3
Figure 3 – SF188 spheroids cultured in the presence of the vehicle (Veh) or the EC50 concentration (14.8µM) of CBD for 5 days show a clear decrease in spheroid size. The box and whisker plot shows the decrease in spheroid size from cells cultured in the absence of CBD (1) to those cultured in CBD (14.8 µM) (2). The portion of the box coloured blue represents spheroids with a volume 25% above the median, and the portion of the box coloured green represents those spheroids with a volume below the up to 25%.
4. Lactate Dehydrogenase Assay
Lactate dehydrogenase (LDH) is a soluble enzyme present in most cells which is released into the culture medium upon cell death due to damage of the plasma membrane. The breakdown of the membrane may be due to either necrosis, or in smaller amounts, apoptosis or autophagy. To date we have this assay on the SF188, BXD-1425EPN and astrocyte cells in both normoxia and hypoxia after 24 hours and 5 days

Figure 4
Figure 4 – LDH assays on SF188 cells cultured in normoxia for 24 hour or 5 days with 15µM CBD (EC50) indicates that after 24 hours this concentration causes approximately 30% cell death not the 50% seen in the metabolism assays, whereas after 5 days in culture the same concentration of CBD led to approximately 58% cell death.
5. Immunofluorescence
Immunofluorescence is the detection of proteins using fluorescent markers. In the project we intend to use the same antibodies used in the Western blot analysis and in the IHC thus confirming the mode of cell death by a further method. Currently, three of the four antibodies have been optimised. Because of the methodology, using cells cultured in chamber slides, the immunofluorescence for the optimised antibodies, LC3B, P62 and Caspase 3, will begin once the Ki67 antibody has been optimised.
6. RNA Analysis
RNA analysis to look at potential gene expression changes caused by exposure to CBD is still to be performed. Any changes in expression can be quantified and may provide multiple pathways which are being effected by the CBD. The changes in RNA expression, either by switching on genes, or switching off genes, could provide key evidence to potential resistance, mode of action and cause of cell death.
RNA microarrays will be used to compare SF188, BXD-1425EPN and astrocyte cells cultured in the presence and absence of CBD for 24 hours and 5 days in normoxia and for the SF188 and astrocyte cells in hypoxia. The cell pellets to be used in this analysis have been collected and will be sent to our collaborating group in the coming weeks.
7. Cell Cycle Analysis
Cell cycle analysis enables the position of a cell through its replication cycle when it undergoes and event to be established. This event may be cell death or it may be that the cell becomes static. We have performed some preliminary experiments on the SF188 and BXD-1425EPN cells to look at this but have not discovered a specific stage at which cell death is more likely to occur. Cell cycle analysis of the astrocyte cells will be performed over the next few months. The more slowly growing cell lines will not be investigated by this means as this would probably not be fruitful.
8. Additional Work and Future Plans
We have begun working with other groups to develop additional experiments such as an ELISA assay to look for immune markers expressed on spheroids which may be released into the cell suspensions as a way to investigate the effects of CBD on immune reactions. We have also held preliminary discussion with another group to look at CBD more specifically with T and B cells (immune cells). In order to pursue this work, additional grant funding would be required.
As previously mentioned we currently have three MSc students working on additional CBD projects.
i. Western blot analysis for apoptosis and autophagy markers. The cells will also be grown in normoxic and hypoxic conditions in the presence of CBD to investigate if this has any effect on levels of apoptosis and autophagy
ii. Currently some patients are taking cannabis oil as an adjuvant therapy for their brain tumour. The treatment is to take the oil for CBD 3 days on, 3 days off. We would like to replicate this in vitro by giving the cells 1µM CBD for 3 consecutive days and then looking at cell viability using viability tests (Resazurin) and Western blotting for apoptosis and autophagy markers. The cells will also be grown in normoxic and hypoxic conditions in the presence of CBD to investigate if this has any effect on levels of apoptosis and autophagy.
iii. Investigate the mechanism of action of CBD in further detail on paediatric brain tumours to see if we can determine the receptors through which CBD is having its action (CB1 or CB2) suing proliferation assays to determine the drug’s effects on the cells.
On behalf of the research team, we would like to thank Brain Tumour Action, Astro Brain Tumour Fund, Amelia’s Appeal, Make William Well and the Jessica Hope Foundation for making this study possible. We are excited about the results and the potential impact we can share with the scientific community later in 2019 and 2020 at the next International Society of Paediatric Oncology Conference.

